- Your cart is empty
- Continue Shopping

Product
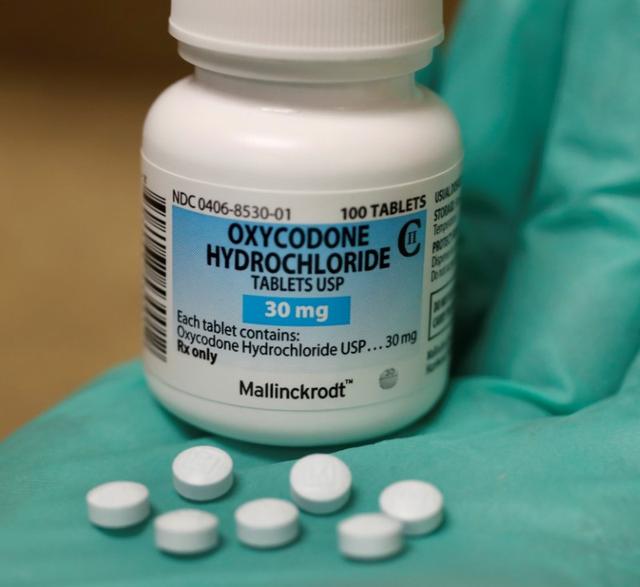
30 M Oxycodone Hydrochloride 30 mg by Mallinckrodt Inc
30 M Oxycodone Hydrochloride 30 mg by Mallinckrodt Inc (Oxycodone Hydrochloride Tablets, USP (CII)) are an immediate-release oral formulation of oxycodone hydrochloride indicated for the management of moderate to severe pain where the use of an opioid analgesic is appropriate.
30 M (Oxycodone Hydrochloride 30 mg)
Pill with imprint 30 M is Blue, Round and has been identified as Oxycodone Hydrochloride 30 mg. It is supplied by Mallinckrodt Pharmaceuticals.
Oxycodone is used in the treatment of chronic pain; pain and belongs to the drug class narcotic analgesics. FDA has not classified the drug for risk during pregnancy. Oxycodone 30 mg is classified as a Schedule 2 controlled substance under the Controlled Substance Act
CONTRAINDICATIONS
- Oxycodone tablets are contraindicated:
- In patients with hypersensitivities to oxycodone.
- Any situation where opioids are contraindicated
- In patients who have or are suspected of having paralytic ileus.
- In patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment).
- In patients with acute or severe bronchial asthma or hypercarbia.
ADVERSE REACTIONS
- Severe adverse reactions: respiratory depression, apnea, respiratory arrest, circulatory depression, hypotension, and shock.
- Common adverse reactions include nausea, constipation, vomiting, headache, and pruritus.
Additional Information
| Dosage | 15 mg, 30 mg |
|---|









Reviews
There are no reviews yet.